Our Silicon lexicon is a collection of facts and vocabulary about silicon. As you might know, Silicon or Si rules as the “second most abundant element on Earth, next to oxygen.”
What is a Silicon Lexicon? And Why?

Silicon Facts: Without Si, It’s Very Possible That The Aeronautics And Space Industry Would Have Never Been Born.
According to Merriam-Webster Dictionary “A lexicon is the vocabulary” of a language or branch of knowledge. So we thought we’d give you a brief lexicon about Silicon. Just for fun and reference.
By no means does this collection of facts cover all there is to know about Silicon. It just covers some fascinating facts you need to know about Si. To clarify, Si is the symbol on the periodic chart for Silicon.
This blog is Part One of a three-part series on this vital element. ER Precision Optical qualifies to bring you this information because we are Germanium and Silicon Specialists. (Special Note: Check out our basics of Germanium blog from a previous blog article.
As we say on our products page, “Silicon is used extensively within the infrared optical range. It’s also known for its strong optical characteristics and hardness. Additionally, Si is a key material of choice for the aerospace and defense industries.
Optical Customers: Demand For Optics
ERPOC has added Si crystal growing to meet the demands of our optical customers.” Thus, we have ample reasons to bring you articles about this unique element.
We have significant crystal growth abilities and facilities here in central Florida. In a future blog, we will be telling you more about our three crystal pullers and our production of optical grade silicon. Likewise, we’ll be telling you more about our “semiconductor or electronic grade, and solar grade Silicon” product.
But for now, let’s go back to basics and investigate this amazing element. You see, over the next year, this blog hopes to bring you articles that cover many facets of optics, from simple to complex. Although silicon makes up a little over 27% of the earth’s crust, many people do not know much about it.
Introducing the Eighth Most Abundant Element in the Universe
Silicon is also the 8th most abundant element in the universe. According to the experts, we discover it at the rate of about 650 parts per million. “It’s the principal element in a type of meteorite called aerolites.” Below, see a list of the common properties of Si. Silicon.
- The Metallic and Non-Metallic Silicon Facts.
Science classifies Silicon as a metalloid. That means it has the properties of both metals and nonmetals. Thus, Si has different forms or allotropes. On the one hand, we see “Amorphous silicon” in the form of a gray powder. On the other hand, crystalline silicon gleams as a gray solid with a shiny, metallic luster.
- Warm Silicon Facts
Si conducts electricity better than nonmetals. However, it is not as good a conductor as metal. That makes it a semiconductor, which is a very good thing. Likewise, Si sports a high thermal conductivity and conducts heat well.
Conversely, opposite to metals, this metalloid is brittle. You would not find it to be malleable or ductile. “Like carbon, it usually has a valence of 4 (tetravalent). However, unlike carbon, silicon can also form five or six bonds.”
Life Facts For Si
Plant and Animals, as well as aquatic organisms, process Si for their skeletons. We find it in healthy human skin, hair, nails, and bones. In fact, we need it in our system to properly “synthesize the proteins collagen and elastin.”
Some people think taking a dietary supplementation of Si increases bone density and reduces the risk of osteoporosis. However, that doesn’t mean you should eat sand.
Silicon Facts Vs. Silicone Facts

Do Not Confuse Silicone Items Like Those With The Unique Element, Silicon.
Silicon and silicone are not the same substances. Many people confuse them. Just keep in mind that Silicon occurs naturally. We label it number 14 on the periodic table. However, silicone is synthetic. Manufacturers create it with silicon-oxygen polymers for many different products. It’s that simple. Yet we hear it used incorrectly every day.
The Surprising Crystal Clear Silicon Facts
Amazingly, Si has the same crystal structure as diamond. As you know, diamonds are made of carbon. And that is the element just above silicon in the periodic table. So, there really is no surprise there, but it’s very interesting. It is surprising that we can grow these silicon crystals right here at ER Precision Optics. ..But that is another story and another blog.
The Purity Facts
As we stated, ultrapure silicon is a grayish silver, solid and glossy sheen. But remember, it is not really metal. Science calls it a metalloid. Thus, Silicon conducts electricity only under certain controlled conditions.
This makes it “very well-suited for the electronics industry.” Sand has a high percentage of silicon. We often see it as the starting material to make those familiar “high-purity silicon wafers.” Those are the wafers on which semiconductor devices are made.
Chances are you’ve seen them as part of everyday life, even if you are not involved in the industry.
Be aware that “Electronic grade silicon must be at least is 99.9999999% pure.” We call this nine-nines or 9N pure. Translated from scientific jargon, this means that “only one in a billion atoms is allowed to be something other than silicon.” We can’t help but think of the old Ivory Soap commercials which touted the soap as 99.9 percent pure! However, in electronic science, this claim must be true and precise.
Silicon Facts from History
In 1954, history announced the first commercial silicon transistor. But thousands of years before, it was used as a “chip.” Primitive man chipped it in the form of hard, Silicon-rich rocks. They found it to be an excellent material to make their sharp-edged tools.
The more modern history of Silicon facts began in 1787 when the French chemist Antoine-Laurent de Lavoisier first discovered it in rocks. Then, in 1800, Sir Humphry Davy silicon mistakenly identified Si as a compound.
Later, in 1811, “French chemists Joseph Louis Gay-Lussac and Louis Jacques Thénard probably prepared impure amorphous Silicon by heating potassium with silicon tetrafluoride. They later named it silicon according to the Latin silex (meteorite).”
Development of Silicon Purity

Jons Jacob Berzelius, The Father of Swedish Chemistry.
Finally, in 1823, the Swedish chemist, Jöns Jacob Berzelius identified Silicon in the form of a metal element by the Swedish chemist. He multiplied his discoveries of this element.
Only one year later, he “extracted amorphous silicon in much the same way as Gay-Lussac,” However, he added to the process by purifying the elemental silicon with repeated cleaning. Then, in the same year, he “heated the silicon oxide powder and the mixture of iron and carbon at a high temperature and obtain the iron silicide.”
Finally, in pursuit of Silicon in its purest form, Berzelius created a special process. He “dry-fired the silicon-fluorine-calcium compound, hydrolyzed the obtained solid, and managed to obtain the pure silicon.” Then, in 1824, in Stockholm, Berzelius “obtained relatively pure silicon in powder form. He did it through a procedure of heating potassium fluorosilicate and potassium.” It is no wonder that history gives the honor of the discovery of Silicon, the element, to Berzelius.
By the way, you might have heard of Berzelius previously in connection with chemistry. Experts rank him with Robert Boyle, John Dalton, and Antoine Lavoisier. And, they were the founders of modern chemistry. …But that, as you might guess, is another story.
Take-Aways from Your Silicon Facts
As stated on our products page, here at E.R. Precision Optical, we focus our Silicon production “on the needs of three growing markets:
- Infrared optics.
- Semiconductor components and
- Solar thin-film targets.
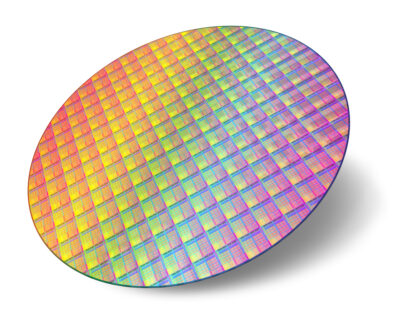
Meet A Silicon Wafer With Processor Cores… Si Carries a Lot of Brains For Our Technology.
But we don’t stop there.” As we previously hinted in this article, we are innovators in this field. We ask potential clients to feel free to challenge us with any” requirement for a hard to find material specification or customized machined components…” And that’s how our story becomes your story in the tradition of Jöns Jacob Berzelius and his discovery of Silicon facts.
Thank you for reading our blog and please return for upcoming details on this fascinating Silicon topic.


